
The compliance challenge
Australia’s aesthetic industry has entered a new compliance chapter. The combination of AHPRA’s tightened cosmetic procedure guidelines, TGA advertising restrictions, and the Queensland Health Department’s S4 medicine controls has placed every clinic under sharper scrutiny. Only a medical or nurse practitioner can purchase and hold S4 stock for a clinic, and they must retain exclusive (or joint, with another prescriber) custody and control. Standing orders are no longer permitted and every vial, dose, and batch must be traceable from delivery to disposal.
For clinics, this isn’t just red tape but a new operational reality. It means airtight custody of stock, accurate records and zero slip-ups in chain of possession.
At DappleOS, our goal was to reimagine how compliance lives inside the clinic workflow. We knew S4 tracking had to be seamless and audit-ready by design. It couldn’t be an add-on or external register, but the foundation for how the platform managed medicines and risk.
Configuring medicines for compliance
The compliance workflow in DappleOS begins with medicines. Setting them up first, ensures that everything linked to them — products, prescriptions and treatment notes — remain clinically accurate and legally sound.
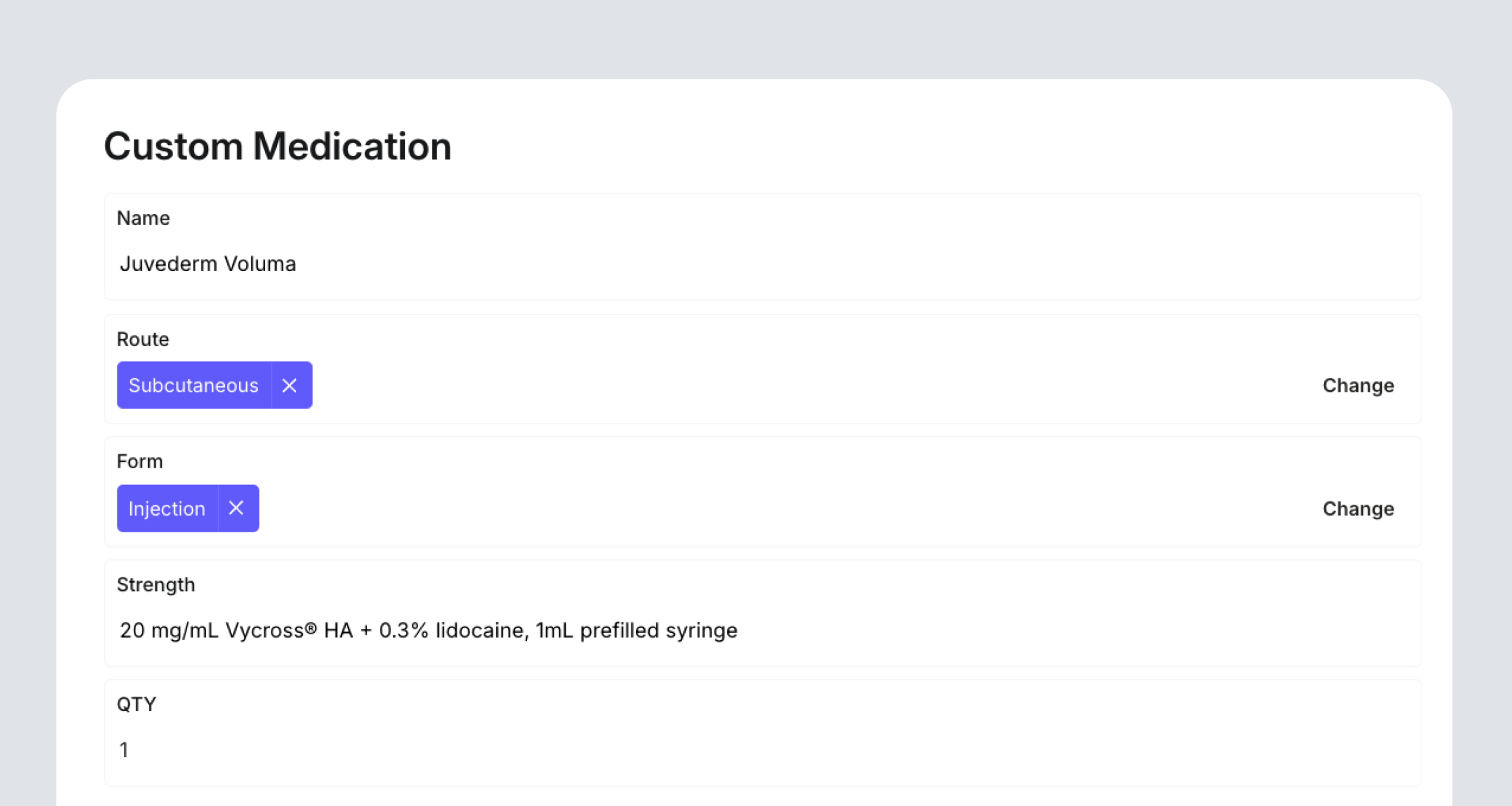
Inside DappleOS, clinics can add Custom Medications such as compounded injectables or private-use formulations. Each medication includes its defining details: name, route, form, strength, and prescribing instructions. It was important that we captured these in the product design to distinguish skincare from a medicine under regulation.
By starting here, clinics create a structured catalogue of approved medicines that can later be linked to products. This keeps prescriptions accurate, makes reporting cleaner, and ensures that every S4 item used in a clinic can be traced back to its source.
Linking products to medicines
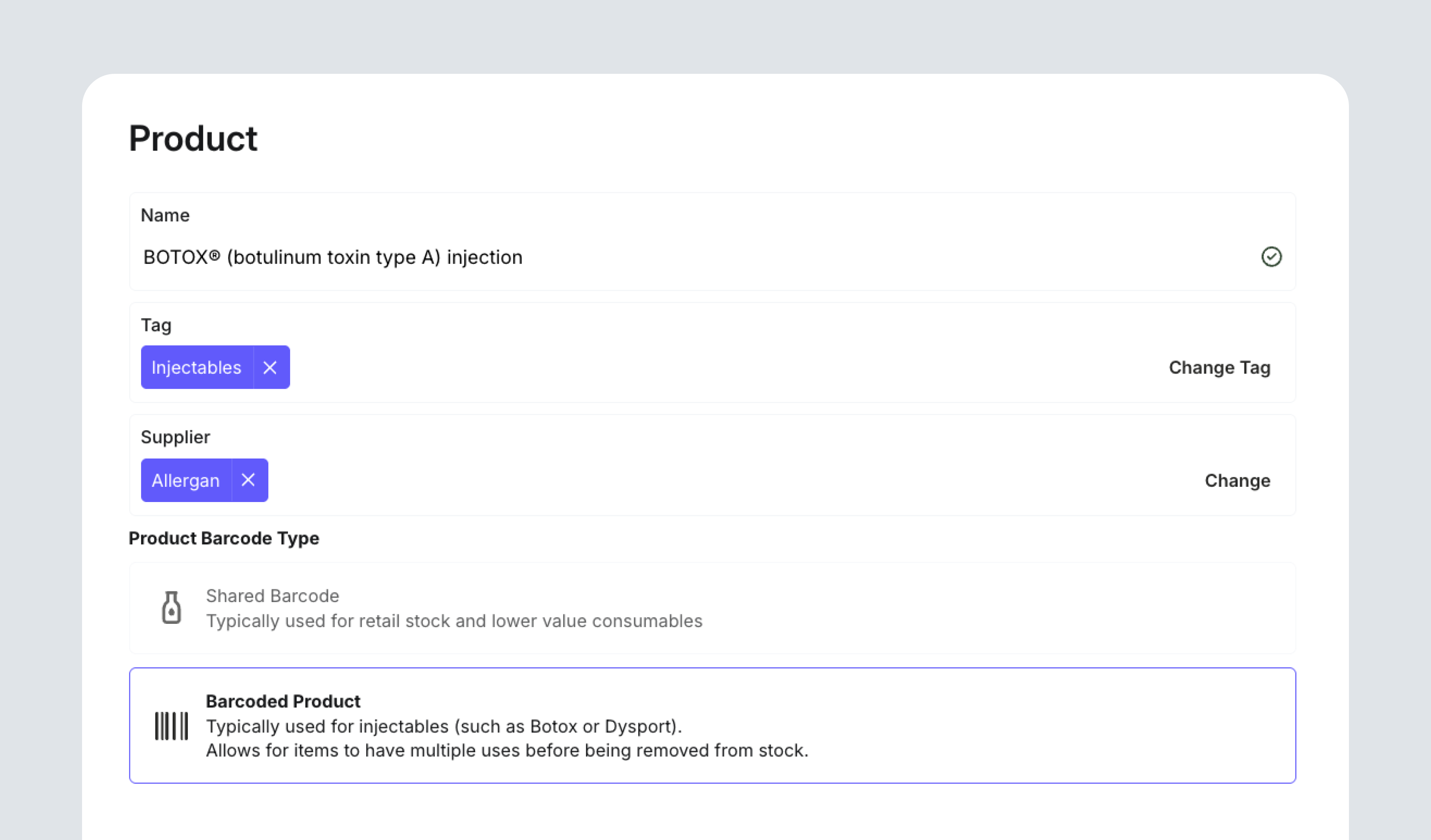
Once medicines are configured, clinics define how those medicines are stored, sold, and tracked. Products can be added using a Shared Barcode (generally for retail or consumables), or a Unique Barcode for Schedule 4 items. Clinics can set additional details such as ordering box size, minimum order quantities, and vial structure.
When a product is marked as Schedule 4, DappleOS unlocks additional compliance settings. The product is linked to a configured medicine record and identified with its ARTG ID and TGA product name. This connection allows the system to manage every S4 item from purchase to administration with precision.
Each vial receives its own barcode, which captures batch, expiry, and use history. This single identifier connects the physical vial, the digital system, and the prescriber responsible for it.
Controlling access through user roles
In DappleOS, permissions form the backbone of compliance. Each user type has clear boundaries for what they can and cannot do.
Prescribers are the only users who can order and receive S4 stock in Queensland. Their details are automatically attached to every purchase order and batch record. Nurses can administer under a valid prescription or direction, and administrative users can view records without being able to change them.
The product team built these access roles into DappleOS to enable clinic alignment with Queensland state legislation on custody and control. This framework prevents unauthorised access and ensures that each user’s actions reflect their professional authority.
Scanning in consultation and live record-keeping
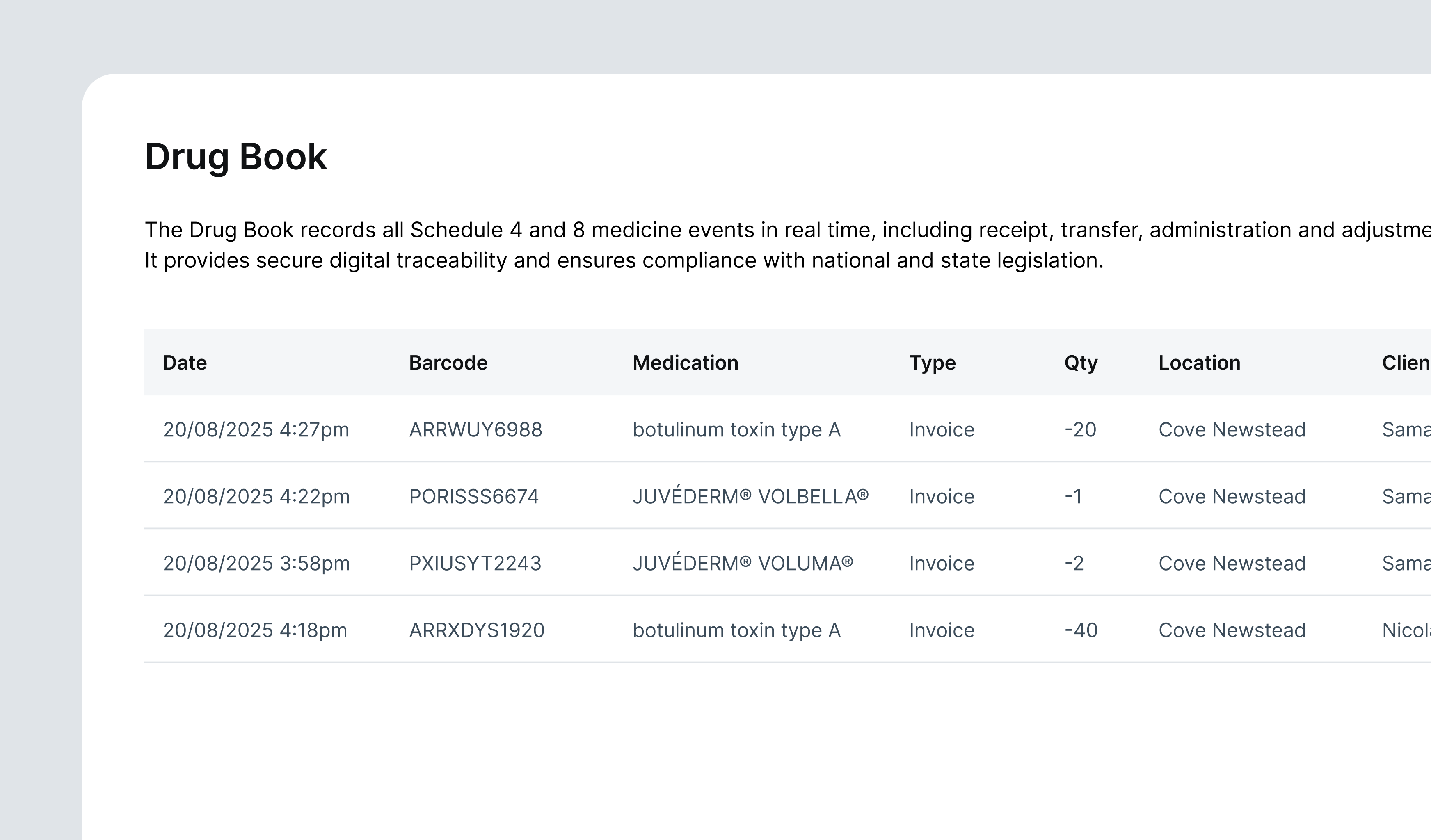
When a clinician scans an S4 vial during treatment, DappleOS captures the prescriber, patient, batch number, expiry, and the amount used. A single scan links all parts of the compliance chain. The patient record updates automatically, the stock ledger adjusts, and the digital Drug Book records the transaction in real time. This digital log provides a complete history of every S4 movement, from the moment a vial is received to the moment it is used.
Why this matters for clinics
The barcode trail reduces variance and removes grey areas around custody. Clinic principles can have confidence that stock and practice are aligned to regulations, especially in jurisdictions like Queensland where exclusive prescriber control is non-negotiable.
Built to keep marketing safe, too
Australia’s advertising rules prohibit promoting prescription-only injectables to the public. DappleOS helps you avoid accidental breaches by separating clinical records from consumer-facing content, and by discouraging the use of S4-referencing terms in templates and workflows that feed public channels.
The outcome
We designed DappleOS to bring structure and visibility to one of the most complex areas of clinical operations. From the first medicine configuration to the final scan in a consultation, every action is captured, linked, and compliant by design.
By uniting medicines, products, roles, and records in a single system, we removed the guesswork from S4 management. Clinics gain the assurance that every vial is accounted for, every prescriber is protected, and every record stands up to audit.
Compliance no longer feels like a burden, rather a competitive edge.
Important: Regulations vary by state and may change. Always follow your local medicines and poisons legislation and Board guidance.




